Iron is a vital nutrient in the human diet. Iron deficiency is the single most common nutritional deficiency worldwide. Distance runners in particular are prone to iron deficiency at higher rates than other athletes. In this article we’re going to discuss why we need iron, how we test for deficiencies, and lastly we’ll discuss treatment.
Disclaimer
This is not meant to replace seeing a physician. Iron is a complicated topic and you can seriously harm yourself. Be smart and get lab work done.
At the Molecular Level
Iron is a key component in two very important proteins: hemoglobin and myoglobin. Hemoglobin is the molecule in red blood cells that carries oxygen from the lungs to the body’s tissues and returns carbon dioxide from the tissues back to the lungs. Myoglobin is found in muscles and transports oxygen from the blood to the muscles. Hemoglobin and myoglobin have very similar structures. Hemoglobin is made up of four subunits each with an iron core. Myoglobin is just one unit with a single iron core.
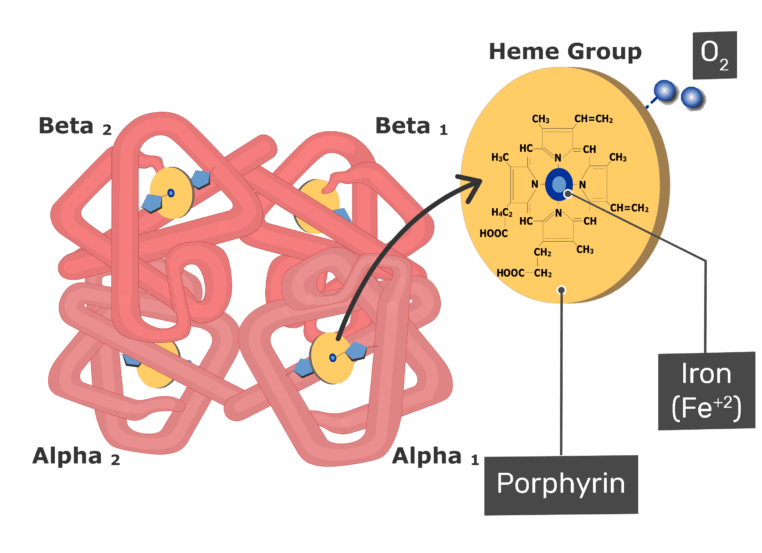
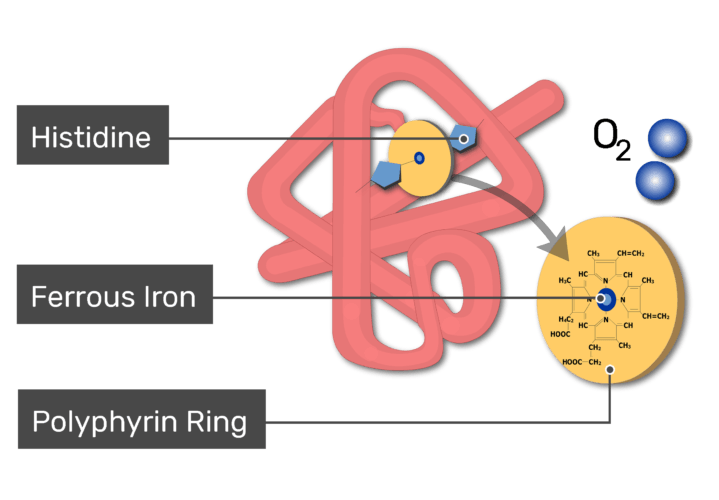
In addition to these two proteins, iron is also found inside your mitochondria and is necessary for making ATP. In fact, the heme iron core that makes up hemoglobin is actually produced in your mitochondria and then transported to your red cells. There is a complex web of biochemistry that links the oxygen your breathe to the ATP your body needs to run. So although we think of iron as something that exists solely for our red blood cells, it’s actually a critical element in our mitochondria as well. Lack of iron can cause dysfunction in both red cells and mitochondria. As you can imagine, this is detrimental to running.
Most people have about 4 grams of iron in their entire bodies. About half of this is locked up in our red cells as hemoglobin. The other half is stored as a substance called ferritin. Ferritin is present in all sorts of cells in our bodies, but most common in bone marrow, liver, and spleen. The liver’s stores of ferritin are the primary physiologic source of reserve iron in the body. We tap into this reserve when our iron needs exceed our iron intake.
Causes of Iron Deficiency
As previously stated, iron deficiency is the most common nutritional deficiency worldwide, including the US. The most common cause of this deficiency depends in part on your gender and age group. For adult women blood loss due to menstruation is the most common cause. For men, blood loss in the GI tract is the most common cause (gastric ulcers, colon polyps, etc). Decreased intake of iron in the diet (vegans, vegetarians) or decreased absorption of iron (celiac disease, etc) is a compounding factor for some.
Why Are Runners at Risk?
It is estimated that, across all sports, 3 to 11 percent of male athletes and 15 to 35 percent of female athletes have some form of iron deficiency. If you zero in on female endurance athletes, that number skyrockets to 50 percent. The exact mechanism by which runners lose iron is a complicated one, and likely multifactorial.
One of the common theories is what’s referred to as foot-strike hemolysis, which basically says that the repetitive strike of your foot against the ground causes the red cells in blood vessels in your foot to pop. Another theory is that runners lose more blood in their stool. Up to 20% of marathoners will have increased blood in their stool at the microscopic level post-race.
The mechanism is complicated, but one proposed mechanism is that the increased demands of the muscles shifts blood away from your intestines causing a mild ischemia to occur, which then results in microscopic levels of blood loss. A similar mechanism causes blood loss in the urine of endurance athletes, with the decreased blood supply leaving the kidneys susceptible to damage. Sweating is yet another mechanism of iron loss. Although sweat is mostly salt and water, iron is present in sweat at a concentration of 1 mg/liter.
Excess iron is toxic to our bodies. We have evolved a complicated mechanism to prevent too much absorption. Sometimes this mechanism works against us. The most interesting theory on iron deficiency in runners involves this mechanism. In particular, a small protein called hepcidin. Hepcidin is a key regulator of the entry of iron into the circulation. Basically, it blocks the absorption of iron. It applies the brakes on your gut’s ability to absorb iron. Levels of hepcidin naturally goes up after hard workouts and inhibits iron absorption, with the worst effects occurring between about three and six hours afterwards. If you take your iron during that window, you may simply not be absorbing it.
To complicate matters hepcidin levels will naturally increase throughout the day independent of exercise, following a circadian rhythm.
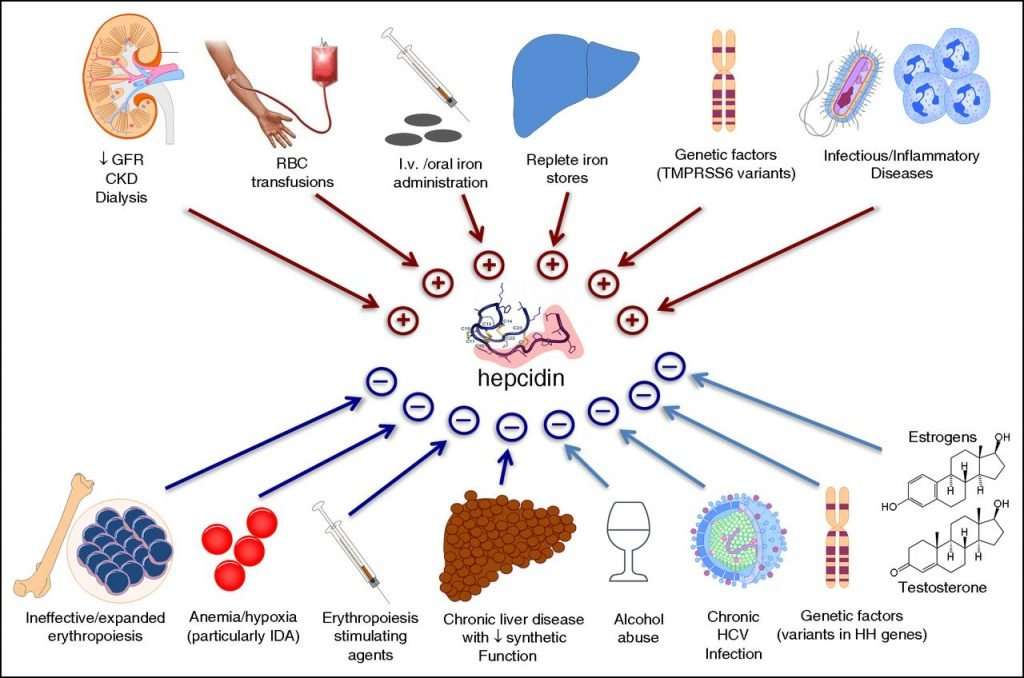
The diagram above highlights the central role hepcidin plays in iron regulation. An entire book chapter could be written on this protein, so I won’t delve any further into how it works. But we’ll discuss hepcidin one more time at the end of this post.
What Are the Symptoms of Iron Deficiency?
There is a wide spectrum of symptoms for iron deficiency, depending on the severity. In very mild cases, there may be no symptoms at all. In general symptoms include:
- Pale skin
- Unexplained fatigue or lack of energy
- Shortness of breath or chest pain, especially with activity
- Unexplained generalized weakness
- Rapid heartbeat
- Pounding or “whooshing” in the ears
- Headache, especially with activity
- Craving for ice or clay
- Sore or smooth tongue
- Brittle nails or hair loss
How Is Iron Deficiency Diagnosed?
It’s important to note that not all anemia is due to iron deficiency. And not everyone that has iron deficiency has anemia. Lots of conditions unrelated to iron can result in anemia, from B12 deficiency to arthritis. For the sake of this article, we’re going to focus on iron. Your physician can order blood work to test for iron deficiency. There are several different indices that are examined during the workup for iron deficiency, and I’ll walk you through them here.
Disclaimer
Keep in mind that lab results need to be interpreted in the context of other lab results, as well as the patient as a whole. One single lab result isn’t particularly helpful, but taken in context, it can help paint a picture of what’s going on. Normal ranges for each test will vary slightly from lab to lab. Some normal ranges vary between men and women, as men naturally have higher red cell counts.
- Red Blood Cell Count (RBC) – a simple count of the number of red cells in your blood.
- Hemoglobin – a measure of the hemoglobin in your blood. A reliable measure of anemia. The normal hemoglobin range is generally defined as 13.5 to 17.5 grams (g) of hemoglobin per deciliter (dL) of blood for men and 12.0 to 15.5 g/dL for women.
- Hematocrit – A hematocrit is a test that measures the proportion of a person’s blood that is made up of red blood cells (RBCs). Your bloodstream consists of RBCs, white blood cells (WBCs), and platelets suspended in a fluid called plasma. The hematocrit is a ratio of the volume of red blood cells to the volume of all these components together, called whole blood. The value is expressed as a percentage or fraction. For example, a hematocrit value of 40% means that there are 40 milliliters of red blood cells in 100 milliliters of blood. Normal levels are generally between 34.9 and 44.5 percent for women and 38.8 to 50 percent for men.
- Mean corpuscular volume (MCV) – a measurement of the average size of a single red blood cell. Iron deficiency anemia causes red cells to become smaller, so the MCV goes down. In contrast, anemia related to B12 deficiency makes red cells get bigger.
- Mean corpuscular hemoglobin (MCH) – a calculation of the average amount of hemoglobin inside a single red blood cell.
- Mean corpuscular hemoglobin concentration (MCHC) – a calculation of the average concentration of hemoglobin inside a single red blood cell.
- Red cell distribution width (RDW) is a calculation of the variation in the size of RBCs.
- Ferritin – we discussed ferritin above as the primary form of iron storage in the body. The small amount of ferritin that is released and circulates in the blood is a reflection of the total amount of iron stored in the body. This test measures the amount of ferritin in the blood.
- Transferrin – this is the main protein in the blood that binds to iron and transports it throughout the body. It goes up when you’re iron deficient as your body tried to transport more iron out of storage.
- TIBC (total iron-binding capacity)—measures the total amount of iron that can be bound by proteins in the blood. Since transferrin is the primary iron-binding protein, the TIBC test is a good indirect measurement of transferrin availability. (Note: Though TIBC is a reflection of the amount of transferrin available, TIBC and transferrin are not synonymous.)
- UIBC (unsaturated iron-binding capacity)—this test determines the reserve capacity of transferrin, i.e., the portion of transferrin that has not yet been saturated with iron.
- Transferrin saturation— dividing the iron concentration by the TIBC produces an estimate of how many of transferrin iron-binding sites are occupied; this is called the transferrin saturation. Under normal conditions, transferrin is typically one-third saturated with iron. This means that about two-thirds of its capacity is held in reserve.
- Serum iron – used to measure the amount of iron that is in transit in the body – the iron that is bound to transferrin in the blood.
The classic presentation of iron deficiency is low hemoglobin (Hg) and hematocrit (Hct). low mean cellular volume (MCV), low ferritin, low serum iron (Fe), high transferrin, high total iron-binding capacity (TIBC), and low iron saturation.
Stages of Iron Deficiency
As we learned earlier in this article, we have iron stores in liver, spleen and bone marrow. When we start to develop iron deficiency, it first strikes our storage of backup iron. Only after our stores take a hit do we start to see true anemia. We think of iron deficiency occurring in 5 stages.
- Stage 1 – Decreased iron stores in your bone marrow, liver and spleen. Your hemoglobin and serum iron tests will come back normal. But your serum ferritin level falls to < 20 ng/mL. The compensatory increase in iron absorption causes an increase in iron-binding capacity (transferrin level).
- Stage 2 – Your body’s ability to make red cells is impaired. Although the transferrin level is increased, the serum iron level decreases and transferrin saturation decreases. Red cell production is impaired when serum iron falls to < 50 μg/dL (< 9 μmol/L) and transferrin saturation to < 16%. The serum transferrin receptor level rises (> 8.5 mg/L).
- Stage 3 – You officially have anemia. Under a microscope, you have fewer red cells in your blood, but they look normal in appearance (size, color, etc).
- Stage 4 – Under a microscope your red cells look small and pale.
- Stage 5 – Iron deficiency affects tissues, resulting in symptoms and signs.
Where Do We Get Iron From?
Iron comes from our diet in two forms: heme iron and non-heme iron. Heme iron is found in meat, poultry, and fish. Red meat contains about three times as much iron as both poultry and fish, making it one of the richest sources of dietary iron. Heme iron is typically absorbed at a rate of 7-35%. Non-heme iron is typically absorbed at a rate of 2-20%. Sources of non-heme iron includes fruits, vegetables, and iron fortified foods like breakfast cereals. Since non-heme iron is absorbed at a lower rate, it often is taken along with vitamin C which assists with absorption. Regardless of which form you take, you can see from the numbers above that the majority of the iron you ingest is not absorbed at all.
How Is Iron Deficiency Treated?
Oral iron tablets are usually a safe, inexpensive, and effective treatment for people with iron deficiency. I will not discuss the exact dosage/duration for treatment, as that is something you should discuss in person with your healthcare provider. But I’ll discuss some generalities.
Certain foods and medicines can reduce the effectiveness of iron tablets. Iron tablets usually should not be taken with food, certain antibiotics, tea, coffee, calcium supplements, or milk. Iron should be taken one hour before or two hours after these items. If you take antacids, your iron tablets should be taken two hours before or four hours after the antacids.
Iron tablets are best absorbed in an acidic environment; taking iron with a vitamin C tablet or orange juice can enhance iron absorption. There are several types of oral iron, and they are all equally effective. For many products, the number of milligrams for the pill is different from the number of milligrams of actual iron molecules (called elemental iron):
- Ferrous fumarate — 106 mg elemental iron/tablet
- Ferrous sulfate — 65 mg elemental iron/tablet
- Ferrous sulfate liquid — 44 mg elemental iron/teaspoon (5 mL)
- Ferrous gluconate — 28 to 36 mg iron/tablet
All of these will provide you with non-heme iron. If you want to take heme-iron without necessarily eating meat, they do sell heme-iron pills. They will provide a higher degree of absorption, but they do cost several times the price of non-heme pills. Of note, they are made of animal products which may violate the dietary choices of the individual. Regardless of which method you chose, it generally takes three to six months to replenish your iron stores depending on the degree of severity.
Can I Simply Cover My Bases and Take Iron — Just To Be Safe?
Iron overload is a serious and life-threatening condition. There is a strong association between iron overload and numerous other ailments, ranging from diabetes and cancer to Alzheimer’s disease. There’s a reason why evolution made it difficult for us to absorb iron.
There exists a gene called HFE that regulates proteins related to iron absorption. Mutations in HFE causes us to absorb more iron that we need. One mechanism by which it does this is decreased production of hepcidin. This takes your foot off the brakes and cranks up your absorption of iron. Each of us has two copies of this gene, one from each parent. Current estimates suggest that more than 30 percent of the U.S. population have one defective copy of HFE. 1 in 200 Americans have two defective copies of HFE — a life-threatening condition called hereditary hemochromatosis. Is one copy of the gene enough to be safe? Studies have shown that people with one defective gene seem to have modest elevations in iron compared to the general population. The prevalence of the HFE mutation is highest in people of northern European descent.
Summary
Iron-deficiency is quite common among runners and have detrimental effects on performance. Seeing your primary care physician is the best way to start the process of diagnosis and therapy. Symptoms should resolve within a few months of therapy.
This article was written by Jay LaPeche, and originally published on reddit. We have republished the article with consent from the author.
Like this?
Let me know by sending me an email at lc@run161.com.
